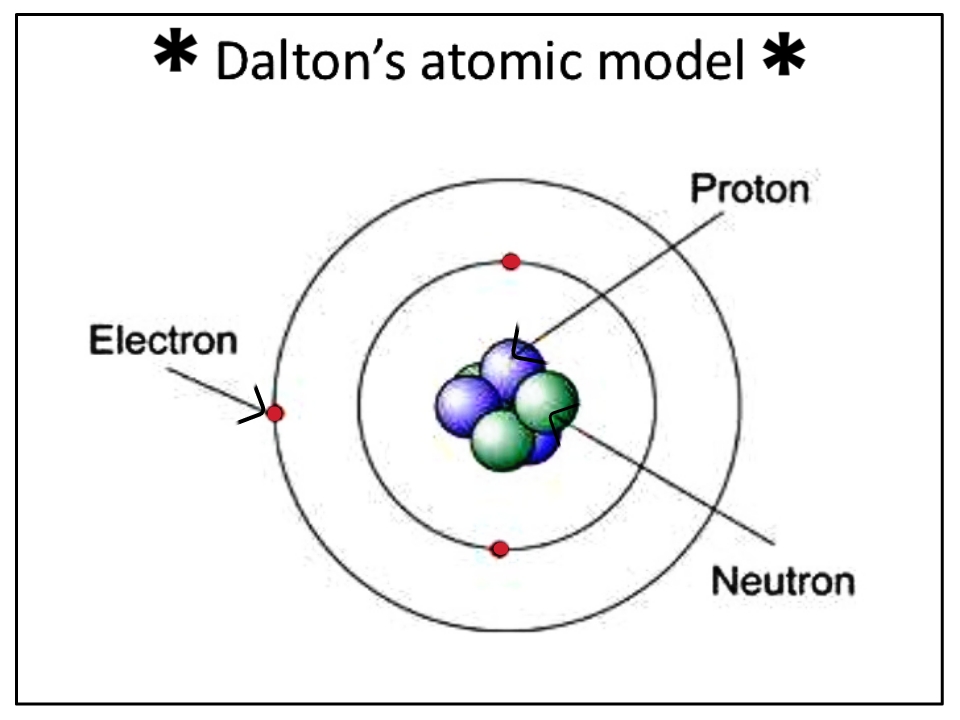
The John Dalton atomic model is a foundational concept in the field of chemistry and physics. This model, developed in the early 19th century by the British scientist John Dalton, laid the groundwork for our modern understanding of atomic theory. Dalton's insights into the nature of matter and the behavior of atoms revolutionized scientific thought and paved the way for future discoveries in chemistry. In this article, we will explore the key elements of Dalton's atomic model, its historical context, and its impact on the development of modern science.
Dalton's atomic theory was put forward in 1803, during a time when the scientific community was beginning to unravel the mysteries of matter. His model proposed that all matter is composed of small, indivisible particles called atoms, which combine in specific ratios to form compounds. This revolutionary idea challenged existing beliefs and provided a new framework for understanding chemical reactions. As we delve deeper into Dalton's atomic model, we'll examine its strengths, limitations, and the subsequent advancements in atomic theory that built upon his work.
In addition to detailing the principles of Dalton's atomic model, this article will also discuss its relevance in today's scientific landscape. By understanding the evolution of atomic theory, readers will appreciate how historical scientific developments continue to influence contemporary research and technology. Join us as we explore the legacy of John Dalton and the profound impact of his atomic model on the world of science.
Table of Contents
- 1. Background of John Dalton
- 2. The John Dalton Atomic Model
- 2.1. Postulates of Dalton's Atomic Theory
- 2.2. Elements and Compounds
- 2.3. Law of Multiple Proportions
- 3. Impact of Dalton's Model on Modern Science
- 4. Limitations of Dalton's Atomic Model
- 5. Evolution of Atomic Theory Post-Dalton
- 5.1. J.J. Thomson and the Electron
- 5.2. Ernest Rutherford and the Nucleus
- 5.3. Niels Bohr and Electron Orbits
- 6. Conclusion
1. Background of John Dalton
John Dalton was born on September 6, 1766, in Eaglesfield, England. He was a self-taught scientist and educator, known for his work in meteorology, color blindness, and, most notably, atomic theory. Dalton's interest in science was sparked by his fascination with the natural world, leading him to conduct experiments and make observations that would later inform his atomic model.
Table of Personal Data
| Name | John Dalton |
|---|---|
| Born | September 6, 1766 |
| Died | July 27, 1844 |
| Nationality | British |
| Field of Study | Chemistry, Physics, Meteorology |
2. The John Dalton Atomic Model
Dalton's atomic model was revolutionary in several aspects. He proposed that atoms were the fundamental building blocks of matter and that each element was composed of identical atoms that differed from those of other elements. This model marked a significant departure from prior theories, which often lacked a clear understanding of atomic structure.
2.1. Postulates of Dalton's Atomic Theory
Dalton's atomic theory is based on several key postulates:
- All matter is composed of atoms, which are indivisible and indestructible.
- All atoms of a given element are identical in mass and properties.
- Atoms of different elements differ in mass and properties.
- Atoms combine in simple whole-number ratios to form compounds.
- In a chemical reaction, atoms are rearranged, but they are neither created nor destroyed.
2.2. Elements and Compounds
According to Dalton, each element is characterized by the type of atoms it contains. For instance, hydrogen atoms are distinct from oxygen atoms. When atoms of different elements combine, they form compounds, which possess properties that differ from those of their constituent elements. This principle laid the foundation for understanding chemical reactions and the formation of new substances.
2.3. Law of Multiple Proportions
Dalton also introduced the law of multiple proportions, which states that when two elements form multiple compounds, the ratios of the masses of one element that combine with a fixed mass of the other element can be expressed as small whole numbers. This law provided a quantitative basis for understanding chemical reactions and further supported Dalton's atomic theory.
3. Impact of Dalton's Model on Modern Science
The impact of Dalton's atomic model on modern science cannot be overstated. It provided a framework for understanding chemical reactions and the concept of elements and compounds. Dalton's work laid the groundwork for future discoveries in chemistry, including the development of the periodic table and advancements in atomic theory.
Dalton's model also influenced other scientific fields, such as physics and materials science. The understanding of atomic structure and behavior has applications in various industries, including pharmaceuticals, energy, and nanotechnology. Dalton's contributions have shaped our comprehension of the natural world and continue to impact scientific research today.
4. Limitations of Dalton's Atomic Model
Despite its groundbreaking nature, Dalton's atomic model had its limitations. One significant limitation was the assumption that atoms are indivisible. Subsequent discoveries, such as the existence of subatomic particles (protons, neutrons, and electrons), revealed that atoms can be further divided. This understanding led to the development of more sophisticated atomic models.
Additionally, Dalton's model did not account for isotopes—atoms of the same element with different masses due to varying numbers of neutrons. These limitations prompted scientists to refine atomic theory and develop more accurate models of atomic structure.
5. Evolution of Atomic Theory Post-Dalton
Following Dalton's pioneering work, several scientists contributed to the evolution of atomic theory, leading to a more nuanced understanding of atomic structure and behavior.
5.1. J.J. Thomson and the Electron
In 1897, J.J. Thomson discovered the electron, a negatively charged subatomic particle. This discovery challenged Dalton's assertion that atoms were indivisible and paved the way for new atomic models that incorporated subatomic particles.
5.2. Ernest Rutherford and the Nucleus
In 1911, Ernest Rutherford conducted his gold foil experiment, which led to the discovery of the atomic nucleus. Rutherford proposed that atoms consist of a central nucleus surrounded by electrons, further refining the understanding of atomic structure.
5.3. Niels Bohr and Electron Orbits
Niels Bohr's model, introduced in 1913, incorporated quantum mechanics to explain the behavior of electrons in atoms. Bohr proposed that electrons occupy specific energy levels or orbits around the nucleus, providing a more accurate representation of atomic structure.
6. Conclusion
In summary, the John Dalton atomic model represents a pivotal moment in the history of science. Dalton's insights into the nature of matter and the behavior of atoms laid the groundwork for modern atomic theory and significantly impacted the fields of chemistry and physics. While his model had limitations, it served as a foundation for subsequent advancements in our understanding of atomic structure.
As we continue to explore the complexities of matter and the universe, it is essential to recognize the contributions of pioneers like John Dalton. We encourage readers to leave comments, share this article, and explore further topics related to atomic theory and its evolution.
Thank You for Reading!
We hope you found this article informative and engaging. Stay tuned for more insights into the fascinating world of science and its history. Your curiosity drives our content, and we look forward to providing you with more thought-provoking articles in the future!